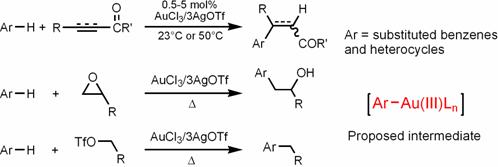
a) Develop gold(III)-catalyzed aromatic C-H functionalization. We independently discovered a gold(III)-mediated hydroarylation reaction of electron deficient alkynes and alkenes. Our group is also the first that developed functionalization of aromatic C-H with epoxides and primary alcohol sulfonate esters. These reactions work at ambient temperature and provide favorable methods for constructing complicated aromatic molecules. Mechanistic studies on some of these reactions were performed and suggested a pathway involving direct auration of arenes by gold(III) to form arylgold(III) species as intermediates. We are in the process of study the scope of these reactions and elucidate the mechanism. We are also investigating other interesting gold(III) chemistry, for instance, redox chemistry and activation of aliphatic alkenes.
b) Discover new silver-mediated oxidation reactions. Oxidation chemistry with high-valent silver ions has not been extensively studied in the past. We have discovered a unique dinuclear silver(I) compound that efficiently catalyzes aziridination of a broad range of olefin substrates. This is the first example of aziridination of unsaturated hydrocarbons catalyzed by silver ions. We propose that high-valent silver species are involved as intermediates in the reaction. Recently we also found that intramolecular amidation of saturated C–H bonds can be catalyzed by the same disilver species.

c) Dioxygen chemistry of silver and high-valent silver chemistry. In industry the epoxidation of ethylene catalyzed by silver particles has been used worldwide to produce ethylene oxide. Dioxygen is used as the oxidant, which makes the process economic and environmentally benign. Although a recent Eastman process further extended this catalysis to butadiene, the scope of this oxidation chemistry is still limited to only two substrates. Industrial preparation of other epoxides, such as propylene oxide, must go through a different, more costly route. The epoxidation reaction has been heavily studied in the solid phase because of its industrial importance, but the silver-dioxyegn solution chemistry has never been explored. Systematic synthesis and evaluation of discrete molecules from reacting silver ions with dioxygen in homogeneous solution are lacking. If these species can be generated in solution their reactivity could be evaluated. Due to high oxidation potential typically associated with silver ions, the dioxygen-derived silver species may exhibit unique oxidation activity. We design and prepare donating, sterically bulky ligands to investigate dioxygen-activation and high-valent silver chemistry.