Thin polymer films modify
surfaces
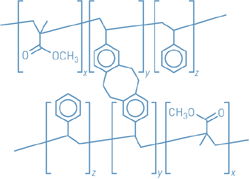 A versatile strategy based on
copolymer cross-linking enables the surfaces of a wide variety
of metals, metal oxides, semiconductors, and polymers to be
modified (Science 2005, 308, 236). Craig
J. Hawker at the University of California, Santa Barbara;
Thomas
P. Russell at the University of Massachusetts, Amherst;
and coworkers prepared ultrathin films of
benzocyclobutene-functionalized random copolymers of styrene
and methyl methacrylate, spin-coated them onto the surfaces,
and then thermally cross-linked the copolymers (shown). The
solvent-resistant films' thickness and surface properties,
such as wetting, adhesion, and adsorption, can be tuned by
changing the chemical composition of the copolymers. The films
adhere to the underlying substrate without chemical bonding,
the authors note. "This generalized route to surface
modification does not depend on specific interactions or
chemistries with the surface," Russell explains. "The
independence from the nature of the substrate is critical for
the fabrication of nanostructured materials for magnetic
storage, field emission devices, and photovoltaic
devices."
A versatile strategy based on
copolymer cross-linking enables the surfaces of a wide variety
of metals, metal oxides, semiconductors, and polymers to be
modified (Science 2005, 308, 236). Craig
J. Hawker at the University of California, Santa Barbara;
Thomas
P. Russell at the University of Massachusetts, Amherst;
and coworkers prepared ultrathin films of
benzocyclobutene-functionalized random copolymers of styrene
and methyl methacrylate, spin-coated them onto the surfaces,
and then thermally cross-linked the copolymers (shown). The
solvent-resistant films' thickness and surface properties,
such as wetting, adhesion, and adsorption, can be tuned by
changing the chemical composition of the copolymers. The films
adhere to the underlying substrate without chemical bonding,
the authors note. "This generalized route to surface
modification does not depend on specific interactions or
chemistries with the surface," Russell explains. "The
independence from the nature of the substrate is critical for
the fabrication of nanostructured materials for magnetic
storage, field emission devices, and photovoltaic
devices."
Neurotoxin made by
most cyanobacteria
Finding
the same toxin being produced by different species of
cyanobacteria is relatively rare, but researchers have now
found a toxin produced by most cyanobacteria. Paul A. Cox at
the National Tropical Botanical Garden in Hawaii and coworkers
find that the neurotoxic amino acid
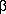
-
N-methylamino-
L-alanine
(BMAA, shown) is produced by 95% of the cyanobacteria genera
and 97% of the strains that they tested, including species
from all five morphological sections (
Proc. Natl. Acad.
Sci. USA 2005, 102, 5074). The samples included
freshwater and marine species of free-living cyanobacteria as
well as cyanobacterial symbionts from lichens and plants. The
authors suggest that under the right conditions, all known
cyanobacteria may be able to produce BMAA, meaning that there
is a chance for widespread human exposure. BMAA, which was
originally discovered in seeds native to Guam, has been
suggested as the cause of a neurodegenerative disease that is
widely found among the Chamorro people of Guam and has been
tenuously linked to Alzheimer's disease.
Screening for effects
on cells
A high-throughput method has been developed for identifying
compounds with promising bioactivity based on their effects on
cells [PLoS Biol., published online April 5,
dx.doi.org/10.1371/journal.pbio.0030128]. The technique,
developed by Kevan M.
Shokat and coworkers at the University of California, San
Francisco, in collaboration with a group at Cytokinetics,
South San Francisco, uses the company's Cytometrix automated
imaging and analysis system to monitor cellular effects of
compounds from small combinatorial libraries. The system
classifies small-molecule-induced changes in cell size and
shape, organelle structure and position, fluorescence stain
intensity, and other observable properties. Shokat and
coworkers used the system to identify a new type of enzyme
inhibitor and probe the mechanism of its enzyme target
(carbonyl reductase 1), revealing a previously unknown role of
the enzyme in apoptosis. By providing a direct window on the
effects of compounds on cells, the technique enhances the
feasibility of discovering novel bioactive agents "in a single
screen with only a limited collection of small druglike
molecules of limited chemical diversity," the researchers
note.
Protein sensor homes in
on lead
A new fluorescent sensor based on a protein from a
bacterium that thrives in a brew of toxic heavy metals has an
unprecedented affinity for lead. In addition to its potential
as a lead detector, this protein, with its unusual
sensitivity, could be used to develop more selective chelating
agents for treating lead poisoning (Angew. Chem. Int.
Ed. 2005, 44, 2). Chuan He
of the University of Chicago and colleagues created the sensor
using a strategy they've devised for detecting other metals
(C&EN,
Jan. 26, 2004, page 41). The bacterium Ralstonia
metallidurans produces a DNA-binding protein, PbrR, that's
been known to be involved in allowing the bacterium to survive
in a lead-rich environment. The group prepared a strand of
duplex DNA that contained the PbrR-binding sequence and a
fluorescent base. When lead ions and PbrR are added to the
mix, the protein complexes with the lead and binds to the DNA
duplex, causing the DNA to distort and the fluorescent base to
fluoresce. In developing the sensor, the authors discovered
that the protein binds lead 1,000 times more strongly than
other heavy metals.
Chiral esters from
ketenes
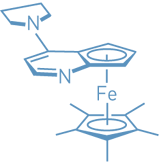 The planar-chiral
heterocyclic compound shown effectively catalyzes the
enantioselective coupling of phenols and ketenes to form
esters, according to a new study (J. Am. Chem. Soc.,
published online April 5, dx.doi.org/10.1021/ja0506152). With the
catalyst, Gregory
C. Fu and Sheryl L. Wiskur of MIT prepare
The planar-chiral
heterocyclic compound shown effectively catalyzes the
enantioselective coupling of phenols and ketenes to form
esters, according to a new study (J. Am. Chem. Soc.,
published online April 5, dx.doi.org/10.1021/ja0506152). With the
catalyst, Gregory
C. Fu and Sheryl L. Wiskur of MIT prepare  -aryl esters from aryl alkyl ketenes and
2-tert-butylphenol in yields of up to 97% and
enantiomeric excesses of up to 94%. The compound has been used
as an enantioselective catalyst for various reactions but
typically acts as a nucleophile, Fu says. In the new
chemistry, the compound is protonated by the phenol; in
protonated form, it serves as a chiral Brønsted acid catalyst.
The ester products themselves are not of primary interest, but
their hydrolysis products are, Fu says. Many
-aryl esters from aryl alkyl ketenes and
2-tert-butylphenol in yields of up to 97% and
enantiomeric excesses of up to 94%. The compound has been used
as an enantioselective catalyst for various reactions but
typically acts as a nucleophile, Fu says. In the new
chemistry, the compound is protonated by the phenol; in
protonated form, it serves as a chiral Brønsted acid catalyst.
The ester products themselves are not of primary interest, but
their hydrolysis products are, Fu says. Many  -arylalkanoic acids, such as ibuprofen and naproxen,
have biological activity, he points out. "It is important to
have good, general methods for the catalytic enantioselective
synthesis of
-arylalkanoic acids, such as ibuprofen and naproxen,
have biological activity, he points out. "It is important to
have good, general methods for the catalytic enantioselective
synthesis of  -arylalkanoic acids, and this work reports progress
toward that objective."
-arylalkanoic acids, and this work reports progress
toward that objective."